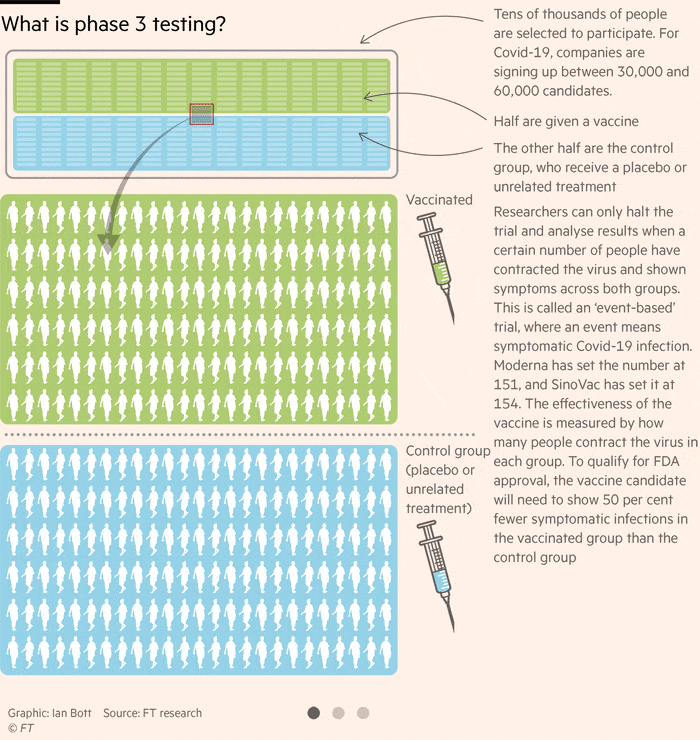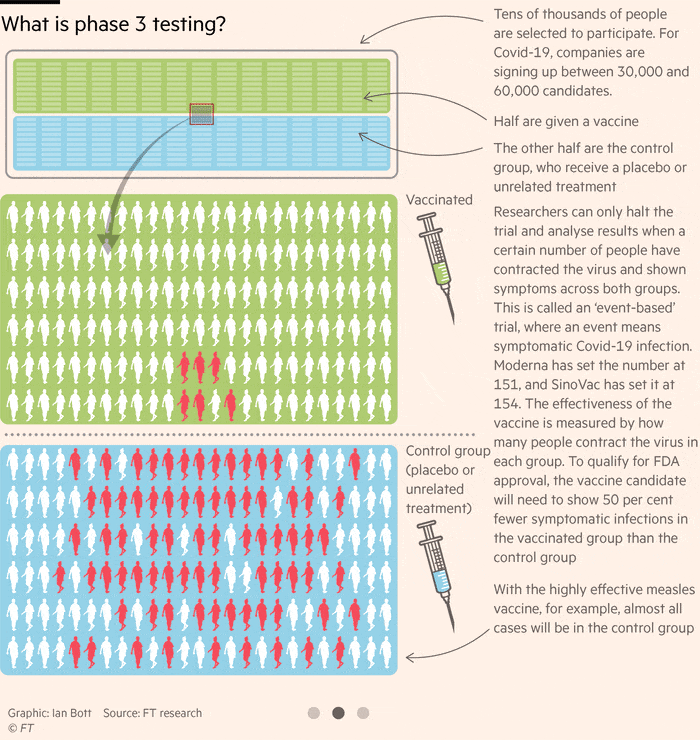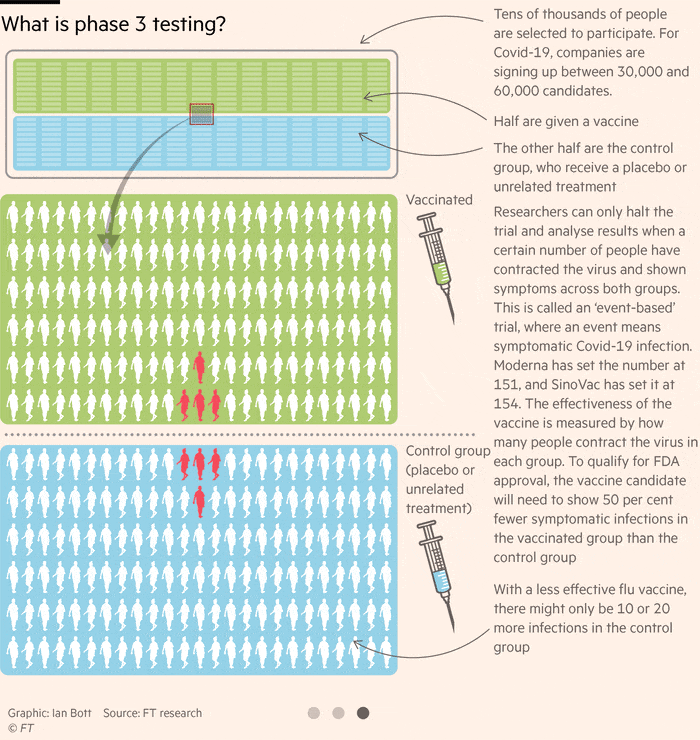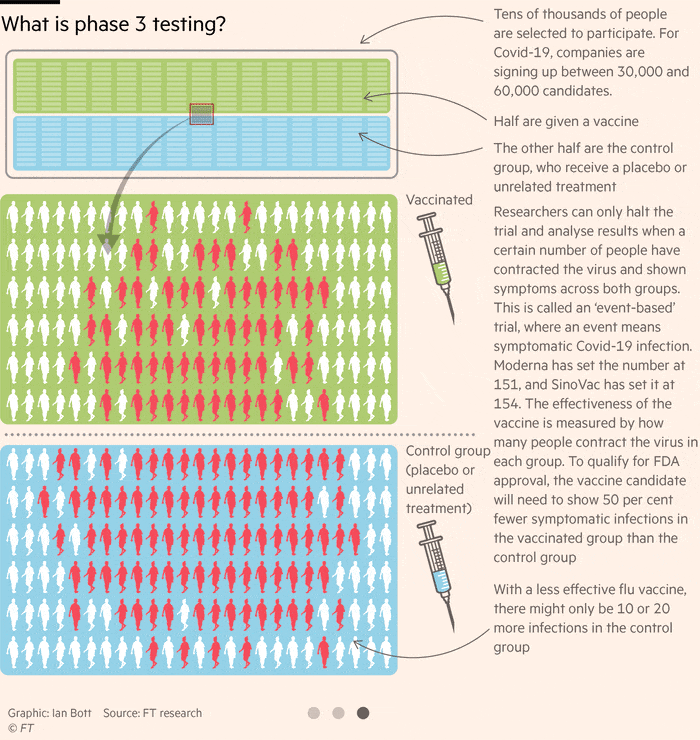
Pfizer Vaccine Update Phase 3

The phase 3 clinical trial of the pfizer biontech vaccine began on july 27.
Pfizer vaccine update phase 3. Pfizer starts phase 3 trial as fauci voices optimism on vaccine timeline. Jansen ph d senior vice president and head of vaccine research development at pfizer inc. The global search for a coronavirus vaccine appears to be taking flight. The start of four phase 3 studies across our portfolio of investigational vaccines is a testament to the talented and dedicated colleagues working throughout pfizer and the continued commitment to unlock the potential promise and value that vaccines hold for our world said kathrin u.
Pfizer and biontech nasdaq bntx started the phase 3 clinical trial for their coronavirus vaccine bnt162b2 around the same time as moderna nasdaq mrna started its phase 3 study for mrna 1273. Pfizer and biontech launched their phase 3 study. A vaccine jointly developed by pfizer and biontech was 90 effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday. The pharma and partner biontech recently asked the food and drug administration for clearance to expand the trial to include 44 000 participants.
Just over 29 000 people had enrolled in the phase 3 study by last friday and as of monday morning 12 000 of them had received the second shot in the two dose regimen used for pfizer s vaccine. Us based pharmaceutical major pfizer and german biotech firm biontech have announced to expand the enrollment of their phase 3 covid 19 vaccine trial to up to approximately 44 000 participants up. A vaccine developed by us based pharmaceutical major pfizer and german biotech firm biontech may be the solution to the. Pfizer hopes to be the first u s.
Third quarter sales dropped by 4 3 hurt in part by lower demand for some of its drugs due to the pandemic.