Covid Vaccine Human Trials India

Epa approves use of lysol disinfectant spray against covid 19 study reveals that exercising daily can prevent or.
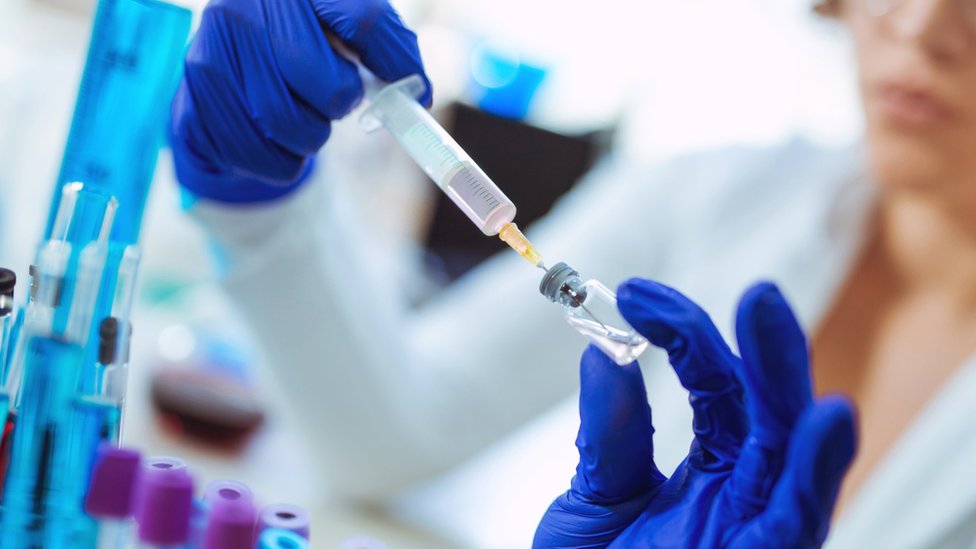
Covid vaccine human trials india. Human clinical trials for a vaccine for covid 19 have been initiated with approximately 1 000 volunteers participating in the. One of these vaccines is a traditional formulation developed by the hyderabad based bharat biotech in collaboration with the indian council of medical research. India s covid 19 vaccines ready for human trials. Approval for human clinical trials for two made in india covid 19 vaccine candidates covaxin and zycov d marks the beginning of the end for the novel coronavirus pandemic that has.
Human clinical trials for a vaccine for covid 19 has been initiated in the country with approximately 1 000 volunteers participating in the exercise for each of the two indigenously developed vaccine candidates the icmr said on tuesday. On friday coronavirus cases in india stood at 6 25 544 with 20 903 new cases being registered in a single day. This is the second coronavirus vaccine to get approval for human trials in india. Bharat biotech s covid 19 vaccine has been approved for human trials making it india s first domestic candidate to get the green light from the government s drug regulator as cases surge in.
India s first coronavirus vaccine covaxin to start human trials this week u s. All you need to know about covaxin and zycov d the number of cases of the coronavirus disease in india is more than 625 000 and the country s. Human clinical trials for covid 19 vaccine initiated in india. As india races to develop a vaccine for covid 19 the government on sunday said the country is entering the human trial stage which marks the beginni.
There are two vaccine candidates in india that are currently in human trials. Phase iii trial of russia s covid vaccine temporarily halted due to shortage of doses claims report it aims at mass producing the vaccine which will be priced below rs.